



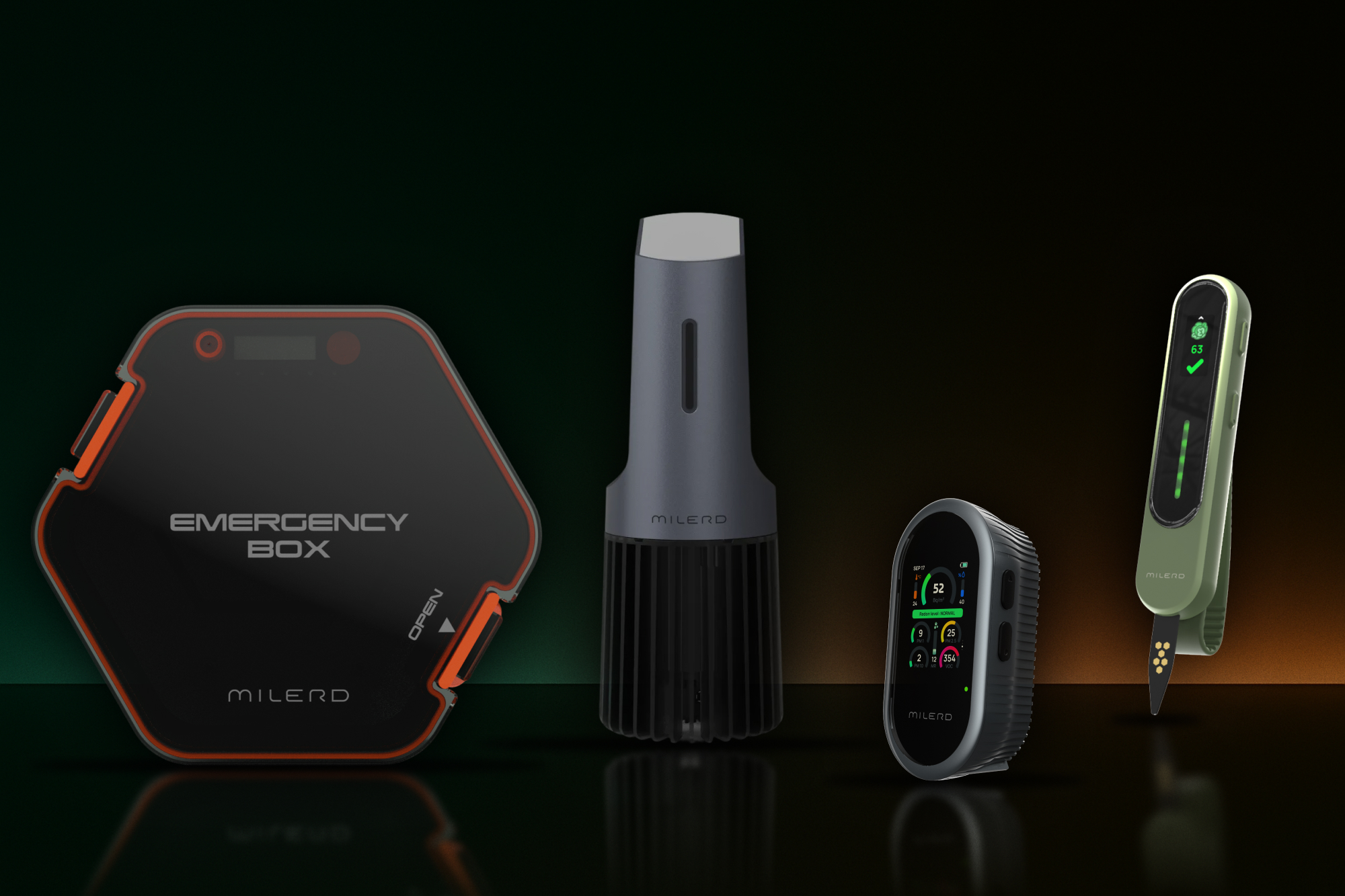
The Gulf Pulse recently featured Milerd in their article "Made in UAE: The innovators building safer technologies," and we're grateful for how they captured what we've built: a complete product cycle operating within a single ecosystem from R&D and prototyping through manufacturing and certification, all rooted in the UAE.
What the piece highlights, and what we're most proud of, is this: genuine engineering depth, speed to market, and unwavering quality control at every stage. These aren't just operational advantages—they're the foundation for sustainable partnerships.
The article notes that we're already developing, certifying, and distributing devices to 20+ countries. But more importantly, it speaks to our strategy: scaling impact through strategic partnerships with distributors, research institutions, and government bodies who share our vision of building safer, healthier, more sustainable communities.
Here's what we believe fundamentally: strong products don't emerge from isolated R&D labs or contract manufacturers. They emerge when engineering and manufacturing work as one unified team, when partners receive not just hardware but a scalable technology platform built to serve their markets and their customers.
We're not just building devices. We're building a platform one that grows stronger with each partner we bring in, each market we enter, each challenge we solve together.
If you're looking for a technology partner who understands the difference between volume and value, between shipping products and building ecosystems, let's talk.
Read the full article: https://thegulfpulse.com/made-in-uae-innovators-building-safer-technologies/


Robotic automation has become the backbone of advanced manufacturing, from welding lines to automated warehouses. Modern industrial robotics systems combine high-power drives, switching electronics, wireless networks and dense sensor grids, creating electromagnetic environments that traditional safety programs were never designed to manage. EMF and radiation safety is now a design parameter for industrial robot technology, not a narrow HSE topic.
Today, industrial robots are no longer isolated in cages. Collaborative systems and mobile platforms work only a few meters from operators and maintenance crews. Every robot used in manufacturing brings not only productivity gains but also additional sources of electromagnetic fields that must be understood, measured and controlled if companies want automation to scale safely across multiple plants.
Most fields in robotic shops come from non-ionizing radiation in industrial environments. High-current busbars, variable-frequency drives, welding power sources and large servo motors generate strong electric and magnetic fields whenever a line runs at full throughput. Close to cabinets and power trunks, magnetic field exposure near industrial robots can spike during acceleration, braking or heavy cutting cycles.
Smart factories add a wireless layer on top. Access points, RFID gates and private cellular networks create wireless communication EMF in smart factories. As plants adopt industrial cellular networks, 5G EMF exposure in industrial IoT becomes relevant for both human exposure and potential interference with sensors or control systems. In high-bay storage and fulfillment centers, radiofrequency exposure in automated warehouses can be significant where scanners, access points and vehicles share the same volume. Fleets of warehouse autonomous robots and AGVs constantly move through aisles, so EMF monitoring for autonomous mobile robots is essential for a realistic picture of exposure over time.
Not all risks are limited to non-ionizing fields. Automated inspection lines can incorporate X-ray or gamma-based systems for inline quality control and material identification. Here, EMF and radiation risk management in industry must cover both shielding design and day-to-day operation: interlocks, access control, maintenance routines and documentation. High-precision reference instruments like the air analyzer for industrial R&D labs help validate shielding concepts and calibrate factory-level sensors. Distributed monitoring becomes more effective than sporadic manual measurements.
For many plants, EMF remains abstract until someone maps it line by line. A structured EMF risk assessment for factory workers turns that abstraction into a clear picture: where fields are highest, who is exposed and under which operating modes. It also shows how EMF interacts with task duration, shift patterns and the presence of workers with medical implants.
A robust EMF program starts with data. Spot checks and area mapping capture EMF measurement in production facilities under realistic operating conditions. For new lines, EMF radiation testing for robotic systems can be included in commissioning so that baseline levels are documented before full-scale operation. Over time, many sites move from occasional checks to continuous monitoring. In dense automation environments, a network of EMF monitoring devices for industrial sites provides real-time insight into how exposure changes with product mix, shift patterns or maintenance work. Combined with industrial EMF dosimetry and surveys, this enables evidence-based decisions on layout changes, shielding projects and process optimizations.
To make continuous monitoring practical, hardware must be designed with industrial integration in mind. A solution such as the Aero Q8 can host radiation sensors, log local data and transmit it to plant systems, while also supporting additional environmental channels where needed. At the same time, companies increasingly look at overall worker well-being, not only EMF. Complementary technologies like the Detoxer device for contamination and process safety help reduce other invisible burdens, such as chemical or food-related contamination in canteens, labs or support areas. Together, targeted EMF monitoring and broader contamination control create a safer and more predictable environment for people who keep automated lines running.
Once EMF has been mapped and quantified, the next question is how it aligns with occupational EMF exposure limits in industry. These limits define what is acceptable for employees during an eight-hour shift and over long careers, and they increasingly influence how robotic cells, charging zones and high-power cabinets are designed. EMF safety standards for industrial robots and related guidance documents translate abstract field strengths into concrete design criteria: where barriers are needed, which areas require restricted access and how long personnel can remain in certain zones. For companies deploying large fleets of robots across multiple sites, EMC and EMF compliance for industrial robots becomes not just a certification checkbox, but a strategic requirement for protecting brand and uptime.
Even when average levels are within limits, some groups need special consideration. Employees with pacemakers, insulin pumps or other implanted devices may react differently to magnetic fields. Defining an EMF safe distance from robotic cells gives them and their managers clear rules: which zones are unrestricted, which areas require time limits and where access should be avoided. Clear EMF zoning and signage in factories turn survey results into something everyone on the shop floor can understand at a glance. Practical instructions for operators, maintenance teams and contractors embed EMF considerations into routine tasks, while refresher workshops based on EMF and radiation safety training for factory staff keep knowledge current and connected to real events on site.
For many OEMs and plant owners, the main challenge is not identifying risks but turning requirements into reliable hardware that can be rolled out across multiple sites. Milerd R&D focuses on full-cycle development for EMF and radiation safety in robotic and automated production lines. That means covering the entire journey: concept definition, feasibility studies, sensor and electronics design, firmware, connectivity, enclosure engineering and compliance testing, all the way to scalable manufacturing.
Because these projects sit at the intersection of safety, automation and IT, the team designs monitoring hardware that matches the plant’s constraints: mounting options for robotic cells, suitable ingress protection, integration with existing fieldbuses or industrial Ethernet and appropriate cybersecurity measures for data flows into higher-level systems. Practical EMF and radiation monitoring in automated plants rarely depends on a single device, so Milerd R&D builds on modular platforms that can host multiple sensors and communication options, allowing the same hardware family to support production lines, warehouses and technical rooms.
To make expectations transparent, Milerd R&D documents its experience in industrial safety case studies with custom hardware and follows a clear sequence of end-to-end R&D stages for OEM hardware projects. For companies investing in robotic and automated lines, this structure reduces uncertainty: they know how EMF and radiation safety devices will be specified, tested and industrialized long before the first units reach the factory floor.


Digital health products and wearable health solutions are rapidly moving from pilots to everyday clinical tools. As digital health technology becomes embedded in care pathways, from continuous remote monitoring to at-home rehabilitation, each new device adds another radio, sensor and processor into an already dense electromagnetic environment. For OEMs and clinics, the question is no longer what is digital healthcare in theory, but how to keep a real digital healthcare ecosystem stable, safe and reliable at scale.
Regulators demand evidence; clinicians expect seamless workflows; patients assume that connected digital medical devices “just work” next to Wi-Fi routers, smartphones and hospital equipment. Poorly planned EMF testing for wearable and digital health devices can lead to costly redesigns, delayed approvals and unpredictable field issues. Milerd R&D supports OEMs and healthcare providers with full-cycle R&D and manufacturing — from early concept and EMF-aware design to prototyping and scalable production — so that new digital trends healthcare and new healthcare technology trends can reach the market with confidence instead of risk.
Health wearables are no longer simple step counters. A modern wearable health device combines sensitive sensors, wireless radios and edge computing, often positioned directly on the patient’s body and used for critical decision-making. Unlike consumer gadgets, digital medical devices are expected to stay reliable in a hospital full of monitors, infusion pumps and imaging systems, and then continue working at home among routers, smart TVs and chargers. In this context, EMF testing for wearable devices becomes a core part of clinical safety, not an optional engineering checkbox.
Every wireless health device interacts with a complex field of radiofrequency and low-frequency signals. Without clear EMF safety standards for wearables and defined EMF exposure limits for medical devices, subtle electromagnetic interference can cause dropped data, false alerts or intermittent freezes that are hard to reproduce. Issues such as Bluetooth medical device interference or EMF exposure from wireless medical devices rarely appear in simple bench tests, but they are quickly noticed by clinicians and patients in real-world use.
For OEMs building home health monitoring solutions, home health monitoring device EMF is just as important as hospital performance. Background EMF in apartments and offices is unpredictable, and devices must remain stable near multiple phones, access points and chargers. Using tools like EMF monitoring in clinical and home environments and an indoor air quality monitor for digital health environments helps teams characterise the real environment around their products and design for wireless health device safety from the beginning.
For OEMs, EMF testing for medical devices is tightly connected to regulatory approval, market access and long-term liability. Authorities expect clear evidence that each product meets defined exposure limits and medical device electromagnetic compatibility requirements across all intended use cases. That means EMF compliance testing for healthcare cannot be reduced to a single lab report: it has to be a structured part of the design and verification strategy.
A robust plan includes EMC testing for digital health devices and EMC testing for medical wearables, as well as targeted EMI testing for medical devices that combine multiple radios and power profiles. Wireless medical device testing typically covers RF exposure testing for wearables and, where applicable, SAR testing for wearable devices, ensuring that both emissions and immunity stay within acceptable limits. Mapping your product to the right digital health device regulatory standards and building a traceable test matrix makes regulatory compliance for digital health devices predictable instead of reactive.
Regulators publish expectations similar to FDA guidance for digital health devices, including EMF testing requirements for FDA approval, labelling and risk management. On top of classic EMC, connected medical device cybersecurity and EMF now form a single discussion: radio interfaces, shielding and firmware updates all influence both interference behaviour and security posture. Milerd R&D reflects this combined perspective when planning verification for new projects and uses experience from completed medical device projects to shorten the path from prototype to approval.
For most teams, the hardest part is not buying equipment, but deciding how to test EMF levels in wearables in a way that actually reflects real use. A practical EMF testing checklist for OEMs starts with the basics: define where and how the device will be used, which radios and power modes it has, and what other equipment will be nearby. This creates a clear profile for IoT medical device EMF testing, whether the product is worn at home, in a clinic, or in mixed environments such as rehabilitation centres and workplaces.
Once the profile is clear, the next step is to map it to standards and build an EMF testing protocol for medical wearables that combines emissions, immunity and exposure tests. Pre-compliance EMC testing for OEMs in a dedicated EMF testing lab for medical devices is often far cheaper than late redesign after a failed certification. For connected devices, remote patient monitoring device testing should include realistic data patterns, wireless charging cycles and worst-case load to identify subtle issues early. Structured documentation of each test run and deviation becomes the backbone for best practices for EMF testing in healthcare and for later regulatory discussions.
In parallel, engineering and clinical teams need a strategy for EMF mitigation for hospital equipment and for the surfaces and tools around patients. Routine measurements and EMF testing services for digital health startups or mature OEMs can be combined with monitoring of surface hygiene and contamination to build a complete picture of the environment. Solutions such as surface and instrument hygiene monitoring help ensure that the same discipline applied to EMF behaviour extends to overall patient and staff safety, making pilots more predictable and easier to scale.

When clinics evaluate new digital health products, EMF criteria should be part of the standard due-diligence process, not an afterthought. Procurement teams can start with a simple EMF risk assessment in healthcare: request clear documentation of the test plan, exposure limits, standards applied and worst-case use scenarios. Vendors should be able to explain how they validated clinical safety of wearable health devices in both hospital and home settings, and provide traceable reports rather than generic marketing claims.
Inside a facility, multiple systems operate in parallel, so EMF safety in hospitals and clinics is closely tied to electromagnetic interference in healthcare environments. Devices worn directly on the body, especially near the chest or head, must be evaluated with implantable medical device EMF safety in mind, covering pacemakers, neurostimulators and other active implants. Clinical engineering teams should ask how the supplier monitors patient safety and EMF exposure over time, and what indicators trigger additional investigation or configuration changes.
Finally, EMF is only one dimension of a safe patient environment. Regular reviews that combine EMF audits with checks of air, water and surface contamination help build a complete protection strategy. Solutions focused on patient environment safety can complement EMF monitoring and give clinics a practical framework for aligning technology choices with overall risk management and quality goals.
For OEM teams, the real challenge is not a single EMF test, but aligning architecture, mechanics and firmware so that EMF testing for medical devices becomes a predictable step in the release process rather than a blocker. Milerd R&D works as a full-cycle partner: we help translate clinical and regulatory requirements into concrete design constraints, select wireless technologies and shielding strategies, and plan verification flows that include IEC 60601-1-2 EMC testing and other relevant standards from day one. This reduces the risk of late redesigns and lets product owners keep roadmaps realistic.
Because the same device must perform reliably in hospitals and at home, our engineers combine lab verification with field-oriented scenarios that reflect best practices for EMF testing in healthcare. Prototyping, small pilot batches and iterative firmware updates allow us to validate exposure levels, interoperability and usability in real workflows before scaling up. For clinics and healthcare networks, this means more confidence that new digital health products can coexist with existing infrastructure and policies around patient safety and EMF exposure.
On the manufacturing side, Milerd R&D maintains the same discipline through scalable production, traceable testing and post-market support. That continuity between R&D, prototyping and industrialisation helps OEMs and clinics treat EMF behaviour as a managed engineering property, not an unpredictable side effect, and launch connected devices that are both innovative and dependable.


For owners and operators of offices, hotels, shopping centers, schools, and mixed-use facilities, air is part of the invisible infrastructure that shapes business outcomes. Radon and other pollutants affect health, absenteeism, and liability, so indoor environmental quality monitoring is becoming a standard expectation. When a commercial indoor air quality monitor with radon capability, an occupant health indoor air quality monitor for high-risk zones, and an indoor air quality monitor for schools and offices feed data into an indoor air quality risk assessment monitor, air quality moves from assumptions to measurable performance.
In commercial properties, radon typically enters through cracks in foundations, service shafts, or technical rooms that are rarely visited but constantly connected to occupied spaces. A radon gas detector for office areas on lower floors is often the first step toward understanding the scale of the problem. Once baseline readings are available, an indoor radon level monitor in representative zones shows how concentration changes across seasons and usage patterns.
For ongoing control, a long term radon monitor tied into a radon monitoring system for commercial buildings provides trend data instead of isolated snapshots. In high-occupancy or high-liability environments, a radon safety monitor for workplace areas becomes part of the same safety infrastructure as fire and access systems. Modern deployments often combine an electronic radon monitor with a low maintenance radon detector in less accessible zones to avoid reliance on manual spot checks.
Continuous measurement also supports mitigation. A smart building radon monitoring strategy can use a smart radon detector with app or a wifi radon monitor to visualize changes after sealing works or ventilation upgrades. A radon mitigation monitoring sensor confirms that remediation remains effective, while a radon compliance monitoring system provides the documented evidence regulators and insurers expect when selecting the best radon detector for commercial use.
Radon is only one part of the air quality picture. Elevated CO₂, chemical pollutants, and moisture generate complaints about “stuffy air” long before limit values are exceeded. A co2 and radon detector or radon and co2 monitor for buildings helps operators see when ventilation no longer matches occupancy, providing a straightforward signal that fresh air delivery should be adjusted.
Sources of chemicals range from furniture and finishes to cleaning products and nearby traffic. A home VOC monitor may be adequate for small spaces, but commercial sites usually benefit from a radon and voc air quality monitor or similar indoor air quality device that tracks VOCs together with other parameters. Moisture adds another layer of risk: a radon and humidity monitor highlights areas where infiltration and dampness coincide, while a radon and mold air quality test kit supports investigations when visual signs of growth or persistent odors appear.
Regulators, investors, and tenants increasingly ask not just whether a building is safe today, but how safety is managed over time. A radon compliance monitoring system with an integrated radon and air quality solution allows owners to present traceable, time-stamped data instead of static reports. For many portfolios, indoor environmental quality monitoring now sits alongside energy and water metrics in ESG policies.

Most programs begin with individual sensors. A co2 voc pm2.5 air quality monitor in a meeting room, an indoor air quality analyzer for technical audits, or an office air quality monitoring device in open-plan areas all provide useful insights. As requirements mature, organizations consolidate measurements into a multi gas indoor air quality monitor that covers particles, gases, and comfort parameters in a single enclosure.
Technical teams need data not just in rooms but also in the air-handling chain. An indoor air quality sensor for hvac and an hvac integrated air quality monitor in supply or return ducts show how air is conditioned and distributed, while industrial sites rely on an industrial indoor air quality monitor in plant rooms and logistics areas. Deployed at scale, these instruments become a network of commercial building air quality sensors that feeds a central view of each site.
The real benefit of monitoring appears when individual devices are connected. Wireless indoor air quality sensors deployed throughout a building can be combined into an iot indoor air quality monitoring system that delivers data to a central platform. When these endpoints act as building management system air quality sensors, operators can link conditions directly to ventilation, filtration, and alarm logic.
A bms compatible radon sensor can trigger targeted responses when thresholds are approached, while a real time indoor air quality dashboard presents conditions in a way that is understandable for technical staff and stakeholders. For distributed portfolios, a remote indoor air quality monitoring solution and cloud based air quality monitoring system provide consistent oversight across all sites.
Under the hood, this depends on dependable logging. Each node effectively operates as a continuous indoor air quality logging device reporting to a platform that functions as an indoor air quality data logger for compliance and long-term optimization.
Many organizations find that off-the-shelf devices only partially match their technical and integration requirements. Milerd R&D focuses on full-cycle development of monitoring solutions: from early research and sensor evaluation through electronics and firmware design, industrial design, and cloud integration to scalable production in the UAE. This approach allows clients to specify how their integrated radon and air quality solution should perform and how it will connect to existing systems.
The process is structured into clear phases, described in the Milerd R&D stages. Depending on the project, the final portfolio may include compact devices for desks and meeting rooms, reference-grade analyzers for commissioning, or robust field units for industrial zones, as illustrated in the Milerd cases.
Radon and indoor air quality monitoring in commercial buildings has moved from occasional testing to continuous, portfolio-wide oversight. By combining accurate sensing, robust hardware, and connected infrastructure, organizations can deploy a commercial indoor air quality monitor network that protects people, supports compliance, and informs better decisions. When backed by a partner able to deliver full-cycle development and production, monitoring evolves from a cost item into a strategic asset that strengthens both building performance and brand trust.


With more health products than ever before, it has never been harder to know what information to trust, which products to choose, and what solutions you really need.
Our mission is to change this by creating the most trusted place to discover curated, quality-assessed health & wellness products from around the world.
Scientific rigor is our guiding principle. Before it goes in your body, it goes through us.
How our curation process works:
Superpower has a dedicated research team that searches the world for the most scientifically rigorous, highest quality, purest, and trustworthy products.
Each year, we review over 10,000 products to carefully select and curate the top 1%.
Our research team uses a combination of qualitative and quantitative algorithms to score brands. We set a high benchmark: only those brands that score a 90% or above meet our standards.
In a world with more options than ever, we’re committed to being the one place you can trust.
Each product then undergoes a comprehensive vetting process over a six-week period. This extensive time window allows us to delve into the details of each standard. Brands are ultimately signed off on by our review committee of leading doctors, scientists, and pharmacologists.
We evaluate products across 11 factors, here are a few of our most important:
Products should have substantial evidence from peer-reviewed studies. We scrutinize the science to ensure the product is safe and effective. We review how long the supporting science has been around, the strength of the scientific advisory board (SAB) behind it, and the quality of the publishing scientific journal.
This also includes assessing the years of data; the number of supported peer-reviewed articles with large sample sizes and proper randomization (double-blind, placebo-controlled); meta-analyses where present; strong support from scientific experts; and whether studies were done primarily on humans vs animals vs in vitro.
Supplements and wellness products are not regulated by the FDA, so we’ve created our own criteria for what’s considered safe versus not with a NO list to avoid supplements with toxic chemicals, microplastics, preservatives, flavors, colors and other provably harmful ingredients.
500+ Banned ingredients
View list
We do the same for personal care and clean home products and so far have banned 500+ harmful ingredients from parabens, phthalates and oxybenzones – so that you know anything on the Superpower marketplace is a product you can trust.
Finally, we look at independent quality testing for purity, strength, identity, and disintegration from the most reputable sources on the internet.

Manufacturing and sourcing standards are key to understanding product quality.
For supplements, we screen for designations such as CLIA, GMP, NSF, and FDA, which together help inform the quality of the manufacturing and sourcing process.
For food products, our screening extends to examining the quality of the ingredients and methods used in production, such as whether the food is grass-fed, processed vegetable oil free, and organic.
Beyond the products themselves, our holistic evaluation looks across the entire supply chain. We perform company background checks and ensure there are not concerns with products or their supply chains.
We have a deep commitment to balancing affordability with quality, and price with performance, ensuring that members receive excellent value in every product they order.
We measure product efficacy not just in numbers but also through the satisfaction of the broader customer universe, aiming for brands that have a substantial collection of genuine, positive customer reviews.
The brand’s mission must supports or mirrors Superpower’s mission to bring preventative care to all and increase human lifespan and healthspan by preventing chronic disease through the pillars of environment, sleep, mental health, physical health, and nutrition.
Once a product is accepted into the Superpower Marketplace, it's not the end of our due diligence.
We reassess products every six months to ensure continued compliance with our brand standards and the latest research.
Ensuring lifelong quality through regular monitoring and re-evaluation.
As science advances, so will the quality of your lifelong health partnership with Superpower – with product recommendations always reflecting the latest in health innovation and research.
Ultimately, we aim to be so confident with all approved products that our medical team would personally use them themselves or recommend them to their loved ones.
Superpower curates the top 1% of health items from around the world, and our research team conducts comprehensive evaluations to ensure top levels of quality, safety, and scientific robustness.
We ensure that our prices are better than anywhere else on the internet and provide them at insider prices to save you money.
We don’t sell our own branded products, we don’t make extra margin on products, and our clinical team is NEVER compensated on sales.
Our promise is to always be scientifically rigorous and 100% incentive-aligned with you


With more health products than ever before, it has never been harder to know what information to trust, which products to choose, and what solutions you really need.
Our mission is to change this by creating the most trusted place to discover curated, quality-assessed health & wellness products from around the world.
Scientific rigor is our guiding principle. Before it goes in your body, it goes through us.
How our curation process works:
Superpower has a dedicated research team that searches the world for the most scientifically rigorous, highest quality, purest, and trustworthy products.
Each year, we review over 10,000 products to carefully select and curate the top 1%.
Our research team uses a combination of qualitative and quantitative algorithms to score brands. We set a high benchmark: only those brands that score a 90% or above meet our standards.
In a world with more options than ever, we’re committed to being the one place you can trust.
Each product then undergoes a comprehensive vetting process over a six-week period. This extensive time window allows us to delve into the details of each standard. Brands are ultimately signed off on by our review committee of leading doctors, scientists, and pharmacologists.
We evaluate products across 11 factors, here are a few of our most important:
Products should have substantial evidence from peer-reviewed studies. We scrutinize the science to ensure the product is safe and effective. We review how long the supporting science has been around, the strength of the scientific advisory board (SAB) behind it, and the quality of the publishing scientific journal.
This also includes assessing the years of data; the number of supported peer-reviewed articles with large sample sizes and proper randomization (double-blind, placebo-controlled); meta-analyses where present; strong support from scientific experts; and whether studies were done primarily on humans vs animals vs in vitro.
Supplements and wellness products are not regulated by the FDA, so we’ve created our own criteria for what’s considered safe versus not with a NO list to avoid supplements with toxic chemicals, microplastics, preservatives, flavors, colors and other provably harmful ingredients.
500+ Banned ingredients
View list
We do the same for personal care and clean home products and so far have banned 500+ harmful ingredients from parabens, phthalates and oxybenzones – so that you know anything on the Superpower marketplace is a product you can trust.
Finally, we look at independent quality testing for purity, strength, identity, and disintegration from the most reputable sources on the internet.
Manufacturing and sourcing standards are key to understanding product quality.
For supplements, we screen for designations such as CLIA, GMP, NSF, and FDA, which together help inform the quality of the manufacturing and sourcing process.
For food products, our screening extends to examining the quality of the ingredients and methods used in production, such as whether the food is grass-fed, processed vegetable oil free, and organic.
Beyond the products themselves, our holistic evaluation looks across the entire supply chain. We perform company background checks and ensure there are not concerns with products or their supply chains.
We have a deep commitment to balancing affordability with quality, and price with performance, ensuring that members receive excellent value in every product they order.
We measure product efficacy not just in numbers but also through the satisfaction of the broader customer universe, aiming for brands that have a substantial collection of genuine, positive customer reviews.
The brand’s mission must supports or mirrors Superpower’s mission to bring preventative care to all and increase human lifespan and healthspan by preventing chronic disease through the pillars of environment, sleep, mental health, physical health, and nutrition.
Once a product is accepted into the Superpower Marketplace, it's not the end of our due diligence.
We reassess products every six months to ensure continued compliance with our brand standards and the latest research.
Ensuring lifelong quality through regular monitoring and re-evaluation.
As science advances, so will the quality of your lifelong health partnership with Superpower – with product recommendations always reflecting the latest in health innovation and research.
Ultimately, we aim to be so confident with all approved products that our medical team would personally use them themselves or recommend them to their loved ones.
Superpower curates the top 1% of health items from around the world, and our research team conducts comprehensive evaluations to ensure top levels of quality, safety, and scientific robustness.
We ensure that our prices are better than anywhere else on the internet and provide them at insider prices to save you money.
We don’t sell our own branded products, we don’t make extra margin on products, and our clinical team is NEVER compensated on sales.
Our promise is to always be scientifically rigorous and 100% incentive-aligned with you.